Ça alors.. 28+ Faits sur Ch4 Polar Or Nonpolar! Here is a list of molecules that are considered.
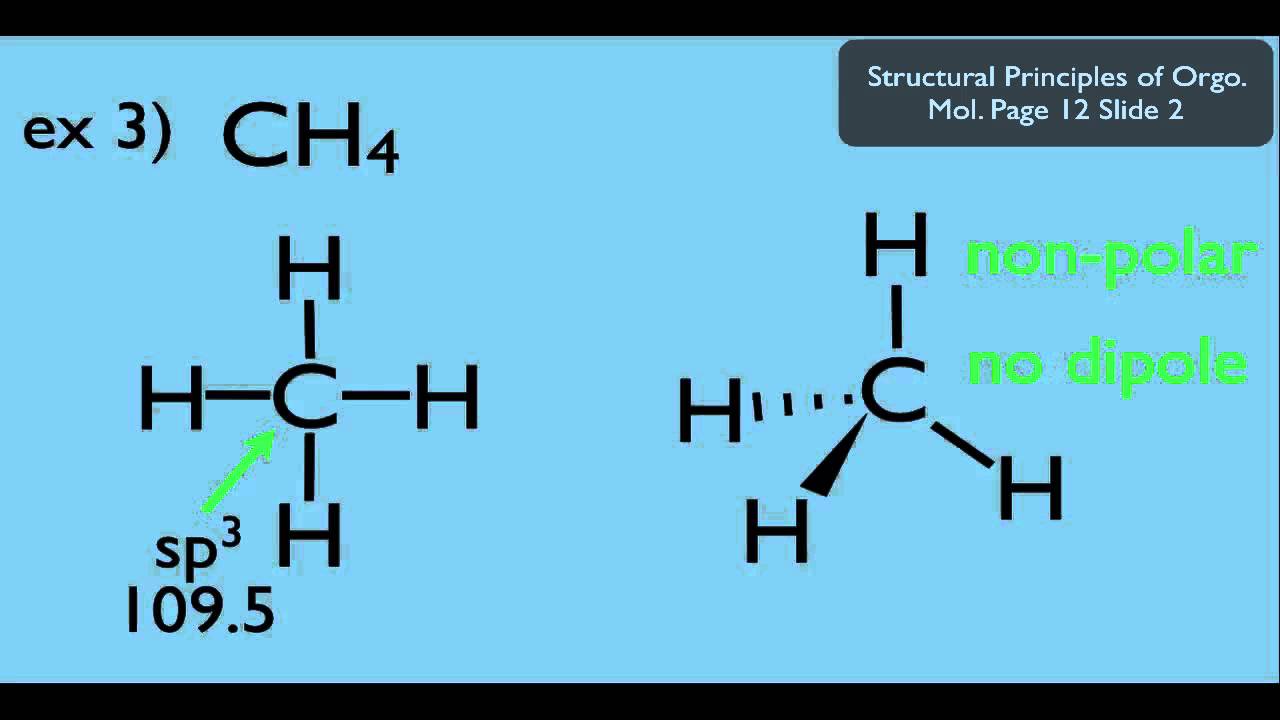
Ch4 Polar Or Nonpolar | H2, n2, o2, cl2, br2, f2, i2 hydrocarbons: Nh3 is polar for the same reason as water. If you look at the lewis structure for ch4 it appears to be a symmetrical molecule. Ch4, or methane, is the same as cf4 in this respect, as are many other molecules made of four halogens surrounding a carbon or silicon atom. Thus, ch4 is a nonpolar molecule.
It provides examples so you can quickly. On the other hand, xef_4 has two lone pairs on xe. Thus, ch4 is a nonpolar molecule. Bir molekülün üzerindeki yük dağılımın simetrik olmaması demektir. Here both carbon and oxygen atoms ( which are considered as geometric centers for this.
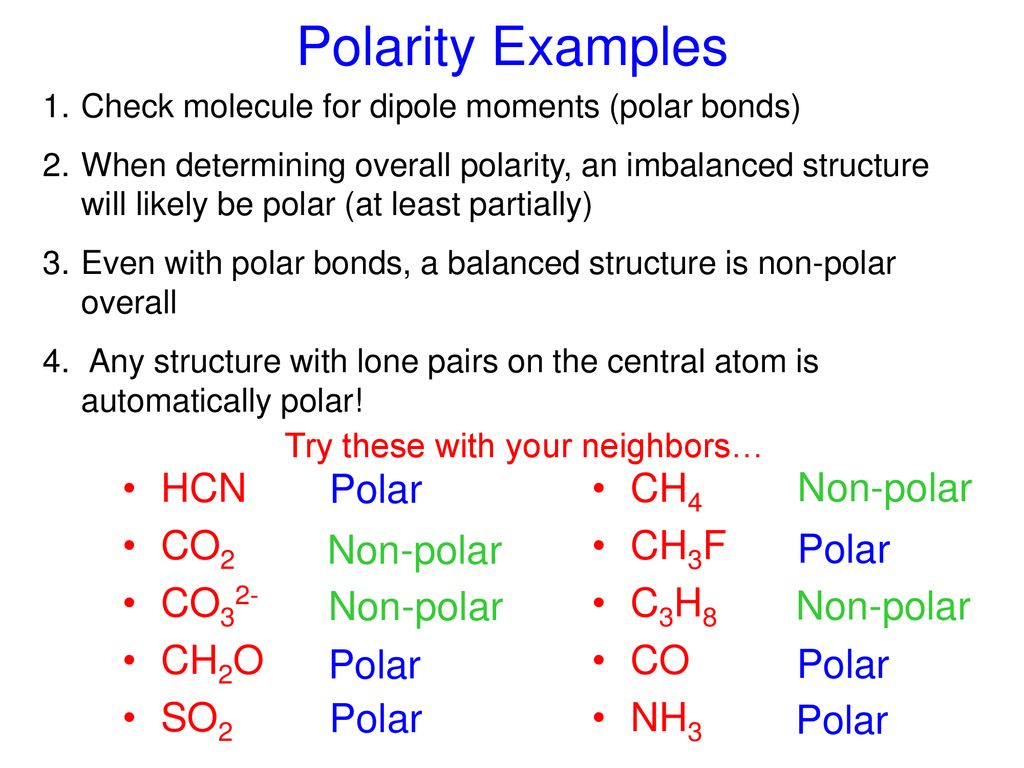
Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each. This video discusses if ch3f is polar or nonpolar. Decide if the following molecules are polar or nonpolar. A lot of students i talk to have questions about solvents, so i've these solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). Although the bonds themselves are polar, the four bonds between carbon and fluorine cancel out one another, generating a nonpolar molecule. Ch4, or methane, is the same as cf4 in this respect, as are many other molecules made of four halogens surrounding a carbon or silicon atom. It provides examples so you can quickly. Ch4, c2h6, c3h8, c2h2, c2h4 identical outer elements with no lone pair on. Feel free to request other molecules that i should be posting about. This video discusses if n2h2, ch3nh2, gah3, and c2f6 are polar or nonpolar using electronegativity and the dipole moments of the bonds in each structure. Ch2 does not exist as a molecule. There are, however, c2h2 and ch4, both of which are nonpolar. If you look at the lewis structure for ch4 it appears to be a symmetrical molecule.
This video discusses if ch3f is polar or nonpolar. Draw the lewis structure first). The shape for ch2cl2 is tetrahedral. Is ch 4 polar or nonpolar? Although the bonds themselves are polar, the four bonds between carbon and fluorine cancel out one another, generating a nonpolar molecule.
The shape for ch2cl2 is tetrahedral. This net dipole moment can be known by noticing the electric charges on the atoms in the molecule. The lewis structure drawing is really misleading because it makes you think that the chlorine atoms are directly opposite from each. I'll tell you the polar or nonpolar list below. There are, however, c2h2 and ch4, both of which are nonpolar. About solvents in organic chemistry. This video discusses if n2h2, ch3nh2, gah3, and c2f6 are polar or nonpolar using electronegativity and the dipole moments of the bonds in each structure. Ch4 and ccl4 have cancelling dipole moments and so are not polar. In chemistry, polarity refers to the distribution of electric charge around atoms, chemical groups, or molecules. Bir molekülün üzerindeki yük dağılımın asimetrik olmaması demektir. A lot of students i talk to have questions about solvents, so i've these solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). Is ch2 polar or non polar? Here both carbon and oxygen atoms ( which are considered as geometric centers for this.
If you look at the lewis structure for ch4 it appears to be a symmetrical molecule. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. Learn vocabulary, terms and more with flashcards, games and other study tools. If you look at the lewis structure for ch4 it appears to be a symmetrical molecule. This video provides a fast way for you to determine if a molecule is polar or nonpolar.

Bir molekülün üzerindeki yük dağılımın asimetrik olmaması demektir. Is brcl3 polar or nonpolar? Submit answer retry entire group 9 mo. The shape for ch2cl2 is tetrahedral. The difference between polar and nonpolar. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal. It provides examples so you can quickly. Submit answer retry entire gr. Feel free to request other molecules that i should be posting about. A lot of students i talk to have questions about solvents, so i've these solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). Is co2 polar or nonpolar? Chemical bonding is the result of either an atom sharing one or more outer orbit methane (ch4). Here is a list of molecules that are considered.
Ch4 Polar Or Nonpolar: Polar protic vs polar aprotic vs nonpolar:
Refference: Ch4 Polar Or Nonpolar
Komentar
Posting Komentar